Monday, December 28, 2015
Thursday, December 24, 2015
Wednesday, December 16, 2015
BLACKOUTS GRAPHITE GRENADES
Explosive Potential of Graphite Dust
Combustible dust is identified as, "Any finely divided solid material that is 420 microns or smaller in diameter (material passing a U.S. No. 40 Standard Sieve) and presents a fire or explosion hazard when dispersed and ignited in air."² The elements of fire include fuel, oxygen and ignition. A dust fire or deflagration occurs when sufficient concentrations of fine particulates are suspended in air and then exposed to a source of ignition such as a spark or welding igniter. This ultimately results in the igniting or combustion of the dust.
THE REST OF THE GRENADE COMPOSITION:
High speed automatic mechanical pressing is commonly used to volumetricaHy load small quantities of primary explosives into blasting caps and detonators and to make small explosive components. Primary explosives may be mixed with graphite to improve flow and antistatic properties, or may be desensitized with waxes, stearates, or polymeric compounds. Secondary explosives and explosive mixtures may be pressed to form booster pellets or to load components directiy as in the case of armor-penetrating projectiles. Where the explosive is too sensitive in its pure crystalline state to permit press loading or lacks the requited mechanical properties in its compressed state for subsequent use, it is coated with polymeric materials such as polystyrene and polybutadiene, to form mol ding powders, often referred to as plastic-bonded explosives. Desensitization is obtained when the explosive crystals are thoroughly and uniformly coated. A typical procedure for making PBX-type explosives involves making a lacquer of a solution of the organic polymer in a solvent, eg, ethylacetate, and a dding it to a water slurry of the explosive. The solvent is distilled off under vacuum while the mix is agitated, precipitating the polymer on the explosive. The coated explosive forms small agglomerates as the solvent removal process continues. It is filtered, washed, and vacuum dried to form a free-fiowing, dustiess, high density powder. Bi- or trimodal size distributions of spherical shaped explosive particles are often used to improve the flow characteristics and packing density of the mol ding powder. Antistatic agents (qv) such as carbon black may be added to prevent dust explosions. In another coating technique, the requited amount of low melting wax is added to a water slurry of the explosive at a temperature high enough to melt the wax. After agitation to distribute the wax on the crystals, the temperature is lowered, the water decanted, and the remaining mass filtered and dried
NON METALLIC COMPOSITES
How are ceramic blade knives detected through airport security
Best Answer: By the metal pins used to attach the handles
If you're sending it through the X-Ray machine, very easily. X-Rays are blocked by things that have high molecular weights. Most ceramic knives are made from Zirconium Oxide. Zr has a molecular weight of 40. Much higher than the 26 of Iron, the principal component of a steel knife. Another common material is Aluminum Oxide, but these are stabilized with Zirconium or Yttrium (39), making them easier to detect.
Beryllium Copper Springs http://www.indiamart.com/hyderabadsprings-components/compression-springs.html#beryllium-copper-springs
the tiny springs are made from Beryllium Copper to eliminate any magnetic signature. Its handle is made from fibre resin and the razor sharp, partially serrated blade is made of 33% glass fibre Nylon 66
Hybrid long glass+carbon fiber reinforced composites http://www.plasticomp.com/hybrid-long-glass-carbon-fiber/
Audi A8 spare wheel recess employs reinforced polyamide http://www.materialstoday.com/composite-applications/news/audi-a8-spare-wheel-recess-employs-reinforced/
Portable, open source Fusion 3D Printer now available for $249, Kickstarter forthcoming http://www.3ders.org/articles/20150907-portable-open-source-fusion-3d-printer-now-available-kickstarter-forthcoming.html
Non-metallic, high-tech materials
Some of the most common non-metallic high-tech materials manufactured and used in the making of knives are:
* black woven graphite composite: so hard it has to be cut with a diamond-tipped blade, using water for a lubricant
* carbon fibre sheet - Immensely strong crystalline filaments of carbon in resin
* celluloid - transparent plastic made from camphor and nitrocellulose
* delrin - a very strong, pure white, plastic material used for knife handles
* kydex - a very strong and sophisticated acrylic-PVC alloy thermoplastic material... ican be heat-moulded, sawn, ground, milled and polished and may also be joined to itself or other materials with an unbreakable bond using a hot glass welding technique
* neoprene a rubber-like compound used as a non-slip knife handle grip
* polyvinyl chloride (PVC) - a durable plastic which can be machined and worked to manufacture knives with standard tools
* zytel - a hard and durable form of nylon used to make many kinds of knives and weapons
* Blackie Collins CIA Folder. There is no steel whatsoever in this spring-assisted, folding lock knife. This assisted opening feature is made possible by a patented internal strut mechanism. The tiny springs are made from Beryllium Copper to eliminate any magnetic signature. Its handle is made from fibre resin and the razor sharp, partially serrated blade is made of 33% glass fibre Nylon 66
Some of the most common non-metallic high-tech materials manufactured and used in the making of knives are:
* black woven graphite composite: so hard it has to be cut with a diamond-tipped blade, using water for a lubricant
* carbon fibre sheet - Immensely strong crystalline filaments of carbon in resin
* celluloid - transparent plastic made from camphor and nitrocellulose
* delrin - a very strong, pure white, plastic material used for knife handles
* kydex - a very strong and sophisticated acrylic-PVC alloy thermoplastic material... ican be heat-moulded, sawn, ground, milled and polished and may also be joined to itself or other materials with an unbreakable bond using a hot glass welding technique
* neoprene a rubber-like compound used as a non-slip knife handle grip
* polyvinyl chloride (PVC) - a durable plastic which can be machined and worked to manufacture knives with standard tools
* zytel - a hard and durable form of nylon used to make many kinds of knives and weapons
* Blackie Collins CIA Folder. There is no steel whatsoever in this spring-assisted, folding lock knife. This assisted opening feature is made possible by a patented internal strut mechanism. The tiny springs are made from Beryllium Copper to eliminate any magnetic signature. Its handle is made from fibre resin and the razor sharp, partially serrated blade is made of 33% glass fibre Nylon 66
CIA Letter Opener
Manufactured from high-tech composite materials with over 60% glass fibres, the CIA Letter Opener's marketing blurb describes it as 'a completely non-metallic knife that provides superior plunging power as well as a hard edge. The scalloped serrations on both sides of the blade give additional opening power on fibrous materials'. For many years the CIA Letter Opener has been described as a lightweight security device capable of being driven through over 12 mm of plywood without breaking. The Cold Steel CAT Tanto, meanhwile, is marketed as being 'black, silent and totally undetectable'. The CAT, as it's known, is made from UV and heat-stabilised, glass-filled zytel nylon. This knife, with its sure-grip handle, reinforced Tanto point and skull-crushing pommel is a super-light, full-size killing tool that is invisible to metal screening devices and would probably pass unnoticed through any security checks. The CAT has all the cutting and penetrating power of its steel sisters. The Cold Steel Vietnam Delta Dart is best described as a vicious weapon designed as a covert operations last-ditch, self-defence tool. The Delta Dart is 8" long and 1/2" in diameter, yet it weighs only half an ounce! The handle is knurled for a positive grip and the butt smooth and rounded, so it's perfect for both thumb and palm reinforced grip positions. The triangular blade geometry gives it incredible puncturing ability. The Delta Dart is made entirely of 43% glass-filled zytel nylon, which is easily sharpened with a nail file. Then there's the Lansky LS17. Although marketed as a general-purpose knife, the LS17 or 'The Knife', as it is sometimes referred to, has become known as a clandestine fighting weapon. The Knife is made from ABS plastic with a 31/2'' double-edged spear point blade with a serrated edge on one side. The non-slip handle has finger grooves and a thumb rest for extra thrusting grip. The LS17 is invisible to metal screening devices.
Manufactured from high-tech composite materials with over 60% glass fibres, the CIA Letter Opener's marketing blurb describes it as 'a completely non-metallic knife that provides superior plunging power as well as a hard edge. The scalloped serrations on both sides of the blade give additional opening power on fibrous materials'. For many years the CIA Letter Opener has been described as a lightweight security device capable of being driven through over 12 mm of plywood without breaking. The Cold Steel CAT Tanto, meanhwile, is marketed as being 'black, silent and totally undetectable'. The CAT, as it's known, is made from UV and heat-stabilised, glass-filled zytel nylon. This knife, with its sure-grip handle, reinforced Tanto point and skull-crushing pommel is a super-light, full-size killing tool that is invisible to metal screening devices and would probably pass unnoticed through any security checks. The CAT has all the cutting and penetrating power of its steel sisters. The Cold Steel Vietnam Delta Dart is best described as a vicious weapon designed as a covert operations last-ditch, self-defence tool. The Delta Dart is 8" long and 1/2" in diameter, yet it weighs only half an ounce! The handle is knurled for a positive grip and the butt smooth and rounded, so it's perfect for both thumb and palm reinforced grip positions. The triangular blade geometry gives it incredible puncturing ability. The Delta Dart is made entirely of 43% glass-filled zytel nylon, which is easily sharpened with a nail file. Then there's the Lansky LS17. Although marketed as a general-purpose knife, the LS17 or 'The Knife', as it is sometimes referred to, has become known as a clandestine fighting weapon. The Knife is made from ABS plastic with a 31/2'' double-edged spear point blade with a serrated edge on one side. The non-slip handle has finger grooves and a thumb rest for extra thrusting grip. The LS17 is invisible to metal screening devices.
The Ace of Spades
The Ace of Spades is a very nasty push dagger made from ABS plastic. It is an extremely robust one-piece construction, capable of massive penetration with its razor sharp, spear point configuration. Of course not all non-metallic knives are manufactured as weapons. There are many companies that use advanced ceramics to create cutting tools and products which combine elegance and strength. Ceramic products using materials such as zirconium oxide and aluminium oxide for applications requiring chemically inert, non-magnetic, non-conductive or non-contaminating materials are also perfect for general applications requiring superior edge retention or wear resistance. They're also ideal for special applications such as EOD work. Many of these weapons have been designed and manufactured for no other reason than to compromise security and endanger life. As I have said many times before in my writings and my training programmes: "You cannot rely on technology alone when it comes to weapons detection, and it makes absolutely no difference whatsoever how much sophisticated state-of-the-art detection equipment you have at your disposal... If you have not trained your personal to understand what kind of weapon they are looking for, what they could be made from or even what they look like, they will not find them!"
The Ace of Spades is a very nasty push dagger made from ABS plastic. It is an extremely robust one-piece construction, capable of massive penetration with its razor sharp, spear point configuration. Of course not all non-metallic knives are manufactured as weapons. There are many companies that use advanced ceramics to create cutting tools and products which combine elegance and strength. Ceramic products using materials such as zirconium oxide and aluminium oxide for applications requiring chemically inert, non-magnetic, non-conductive or non-contaminating materials are also perfect for general applications requiring superior edge retention or wear resistance. They're also ideal for special applications such as EOD work. Many of these weapons have been designed and manufactured for no other reason than to compromise security and endanger life. As I have said many times before in my writings and my training programmes: "You cannot rely on technology alone when it comes to weapons detection, and it makes absolutely no difference whatsoever how much sophisticated state-of-the-art detection equipment you have at your disposal... If you have not trained your personal to understand what kind of weapon they are looking for, what they could be made from or even what they look like, they will not find them!"
All you need to know about PS5
PS5is a nationally-recognised, specialist security consultancy and training provider to the law enforcement, defence and security industries worldwide. The company's training wing 'REACT' delivers highly specialised training protocols to professionals operating across the private and public sectors with specific focus on weapons awareness and personal protection from violence, aggressive behaviour and terrorism. In order to carry out certain aspects of its work, the company has been granted a UK Government Home Office Authority directly approved by the Secretary of State under Section 5. Due to the highly sensitive and sometimes restricted nature of PS5's work, and in order to maintain a high level of security and confidentiality, the company has its own in-house, fully comprehensive, design, photographic and video production capability. This department's primary remit is the design, production and publication of training and educational material as well as the creation of the company's own corporate in-house journal, PCW Review. This PS5 publication is distributed to law enforcement and security professionals in over fifty countries. PS5 also designs, develop and produce a range of security related safety products and training aids.
PS5is a nationally-recognised, specialist security consultancy and training provider to the law enforcement, defence and security industries worldwide. The company's training wing 'REACT' delivers highly specialised training protocols to professionals operating across the private and public sectors with specific focus on weapons awareness and personal protection from violence, aggressive behaviour and terrorism. In order to carry out certain aspects of its work, the company has been granted a UK Government Home Office Authority directly approved by the Secretary of State under Section 5. Due to the highly sensitive and sometimes restricted nature of PS5's work, and in order to maintain a high level of security and confidentiality, the company has its own in-house, fully comprehensive, design, photographic and video production capability. This department's primary remit is the design, production and publication of training and educational material as well as the creation of the company's own corporate in-house journal, PCW Review. This PS5 publication is distributed to law enforcement and security professionals in over fifty countries. PS5 also designs, develop and produce a range of security related safety products and training aids.
Saturday, October 17, 2015
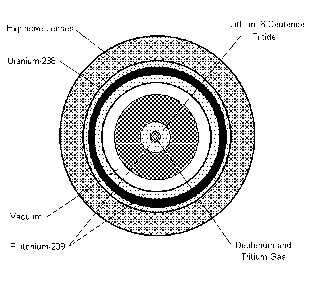
DIRTY BOMB PART IV
FOR A DIRTY BOMB ITS NEEDED 100 UNIT CURIES, AS YOU SEE THIS GOODS HAVE 1.0 UNIT CURIE, NEVERTHELESS IT ONLY WORKS IF ACHIEVES VERY HIGH TEMPERATURES BESIDES THE PETN ITS ALSO NEEDED ETHYLENE GAS.
FOR SEARCH FOR THIS KIND OF PRODUCT IT HAS TO BE SEEN ON THE FEATURES FOR "SENSING TYPE"
THE TUTORIAL HERE:
http://www.instructables.com/id/How-to-Obtain-and-Extract-Americium/step6/How-to-Obtain-and-Extract-Americium/
Monday, October 5, 2015
RED MERCURY DIRTY BOMB PART III (BASEBALL SIZED)
Red mercury, also known as ‘cherry red’ because of its color, is a semi-liquid compound of pure mercury and mercury antimony oxide. It could be used to make a baseball-sized neutron bomb capable of killing everyone within 600 meters (approximately 0.37 mile) of the explosion."
"In the early 1990s, Delta-G made a cash purchase for mercury from
another former state-owned company, Thor Chemicals, a SADF front company that was involved in the network of corporations working to provide materials for the covert CBW program. What this mercury was used for remains unknown. The prosecutor in the Basson trial investigated this purchase since mercury can also be used for the production of sassafras to produce Ecstasy. Others speculate that this purchase was related to the production of Mandrax. However, mercury produced by Thor Chemicals has also been linked to the mysterious nuclear substance"
"eading to the production of so-called red mercury (RM 20/20, chemical formula Sb2O7Hg2), the basic material for producing cheap, miniaturized neutron bombs. Red mercury is a kind of highly thick gel. It serves as the detonator for a small amount of tritium (super-heavy hydrogen), which when exploding produces a wave of pulsing neutrons....the price of a kilogram of RM 20/20 exceeded $400,000...of so-called red mercury (RM 20/20, chemical formula Sb2O7Hg2), the basic material for producing cheap, miniaturized neutron bombs"
Introduction
Ballotechnic materials are materials that react very energetically when subjected to a shock, without losing their solid (or liquid)1 form. Unlike common high-order explosives, these materials do not release hot gases, but rather stay solid when they release this energy. An often discussed example of this is red mercury.
Red mercury is a type of mercury salt, with the formula Hg2Sb2O7, which is then irradiated in a nuclear reactor. It is claimed that this substance has a densityof over 2.0x104 kg m-3, which is extremely high, especially for an oxide. And, as mentioned, it is a ballotechnic material, which means it releases unimaginable amounts of energy when subjected to a shock.
Great, but why is this interesting?
The main reason why this is so interesting is that if red mercury does what it claims, it can be used in the construction of powerful hydrogen bombs. A normal hydrogen bomb works by using a fission explosion to compress a deuterium-tritium mixture so it ignites by fusion. This is rather inconvenient, as created by afission explosion is not something one does with stuff found in the shed. Deuterium and tritium are very easy to obtain, although I won't give pointers how. The idea is to use red mercury, or another ballotechnic material to create the high temperatures and pressures necessary for fusion. Such a device is called aballotechnic nuclear bomb, and if were to exist, it would be as dangerous and powerful as it would be small.
The terrorists will kill us all!
The idea of a nuclear bomb that is constructed from readily-available materials plus some red mercury and that is as easy to conceal as, say, the kilos of drugsthat are readily smuggled into every country of the world, would no doubt make Evil People very happy. There is, unfortunately for them, a small if: all of it depends on the magic of our ballotechnic material. I will now try to show you why the existence of a ballotechnic material is not very likely, based on the above claims. There is little info available on how such a ballotechnic material would do its thing, so I'll give two possible scenarios. I am a physicist, but not really an expert in explosives, so it could be I missed an elaborate explanation for this phenomenon.
Or won't they?
A ballotechnic material, as mentioned, is characterized by releasing enormous amounts of energy while remaining solid when subjected to a shock. Releasing lots of energy when being subjected to a shock is hardly an uncommon property of materials, in fact, that's how explosives work. The shock breaks molecular bonds, and the material turns into a hot, high-pressure gas due to the heat released.
However, a ballotechnic does not turn into a gas, at least, not instantaneously. It is claimed to be capable of holding its solid state while being heated to fantastic temperatures, while having its chemical bonds ripped to shreds and reorganized. This is very unlikely. Furthermore, the temperature needed to produce fusion is many millions of K, not something even remotely reachable with the energy density in the chemical bonds in solids, not to mention the fact that a solid will turn into a rapidly expanding plasma in nanoseconds when subjected to such temperatures. This can be readily seen when one considers a typical chemical bond represents an energy of a few electronvolts, which corresponds to a temperature of a few tens of thousands K-far less than the millions needed for fusion.
Slightly less unlikely is the suggestion that the the energy is nuclear in origin. It is, however, consistent with the fact that the suggested material, red mercury, is neutron-rich and therefore probably unstable. This does ignore the fact that nuclear reactions tend to require enormous amounts of energy to trigger, orders of magnitude more than conventional explosions. Furthermore, this material will also not remain solid when subjected to the kind of temperatures needed to initiate fusion.
Apart from the physical argument, there is a second argument which may be more appealing to the lay person. A normal scientific discovery is published inscientific journals or maybe a conference. Especially in the former case, they are scrutinized by the editor, and subsequently by one or more peer reviewers. The more outrageous the claim-and ballotechnic properties are quite outrageous-the more serious the scrutiny. A search for papers revealed exactly one serious hit, and that is a theoretical study rather than experimental evidence. One would expect that something as shocking as a ballotechnic material would receive more coverage, in respected journals, if it were real.
The fact there is little if any peer-reviewed evidence, the fact that it seems to fly into the face of conventional physics and the fact the claims are quite wild, but lack detail, suggest that this is not a real material, but rather, a scare tactic. The fact that there are a lot of things written in nonscientific publications on red mercury, but nothing in scientific publications 2 is almost the litmus test for a scientific hoax. The main propenent of the existence of these materials, Sam Cohen, the father of the neutron bomb, stands alone in his opinion, and his opponents include physicists such as the great Edward Teller, who calls it "nonsense".
Conclusion
If they exist, the properties of ballotechnic materials make them very suitable for the creation of small but very potent nuclear weapons. However, as their properties seem to defy the laws of physics, and there is no evidence whatsoever, despite the attention to the subject, it is almost certainly a hoax.Sources:
- http://www.quackgrass.com/roots/ddp95.html
- http://chemistry.about.com/cs/chemicalweapons/f/blredmercury.htm
- http://www.nti.org/e_research/e3_42a.html
2: If we exclude the red mercury salt HgS, which has a nice red color but has no claimed ballotechnic properties
http://everything2.com/title/ballotechnic+material
Sunday, October 4, 2015
PART II RED MERCURY DIRTY BOMB (forget the plutonium and the uranium !)
The lenses were cast in special molds made from cellulose acetate. Each lens consisted of two types of explosive, one fast-detonating Composition-B [60% RDX, 39% TNT, 1% wax] and the other slow-detonating Baratol. Each lens had three pieces: two made of high velocity explosive, and one of low velocity explosive. The outermost piece of high velocity explosive had a conical cavity in its inner surface into which fitted an appropriately shaped piece of slow explosive. When the lenses were in place, the fast-detonating part of each lens touched the layer of explosive
http://www.globalsecurity.org/wmd/intro/nuke-he.htm
http://www.globalsecurity.org/wmd/intro/nuke-he.htm
RED MERCURY DIRTY BOMB
Preparation of Lithium Aluminum HydrideIntroduction
When lithium hydride is treated with an ether solution of aluminum chloride under the conditions described in the experimental part of this paper, the new ether soluble compound, lithium aluminum hydride, LiAlH4, is formed according to the equation:
Addition of further quantities of aluminum chloride yields an etheral solution of aluminum hydride:
The latter solution is not stable; it soon deposits a white solid in which the atomic ratio of aluminum to hydrogen still is 3:1, but from which the ether cannot be completely removed without loss of hydrogen. Lithium aluminum hydride, on the other hand, may be freed from the solvent completely by evaporation of the latter under suitable conditions. Through the use of lithium aluminum hydride, new methods, far simpler than any hitherto available, have been developed for the preparation of hydrides such as silane and stannane and of their partially alkylated derivatives. The types of reaction by which these results have been achieved are illustrated by the equations:
LiAlH4 + 2 (CH3)2SnCl2 =======> LiCl + AlCl3 + 2 (CH3)2SnH2 LiAlH4 + (CH3)2Zn =======> LiAl(CH3)2H2 + ZnH2
Reactions such as these usually proceed smoothly at room temperature, and in general give excellent yields of products of high purity.
In the field of organic chemistry, lithium aluminum hydride has already proved extraordinarily useful as a reducing agent. One mole of carbon dioxide is quantitatively absorbed at room temperature by an ethereal solution containing two moles of the new compound; among the products of the reaction are lithium and aluminum salts which yield formaldehyde when they are treated with acids, but the course of the reaction has not yet been completely elucidated. Aldehydes, ketones, acid chlorides and esters are reduced to alcohols, nitrites to amines, aromatic nitro compounds to azo compounds. In many cases in which the customary reducing agents require high temperatures or pressures and give poor yields, the new reagent reacts at room temperature and at an easily regulated, convenient rate, and gives almost quantitative yields. Its action is often highly specific. In the reduction of the nitrites so far studied by us, only primary amines were produced. Its usefulness is enhanced by the fact that olefinic double bonds are not attacked, except in special cases, and it is thus possible to accomplish the selective reduction of various functional groups in unsaturated compounds.
It is of interest to compare the properties of lithium aluminum hydride with those of lithium borohydride. Both compounds are white solids, stable in air at room temperature. Thermal decomposition of lithium borohydride becomes appreciable at from 250°C to 275°C, and seems to involve an intermediate reversible step in which a compound LiBH2 is formed. The decomposition of the aluminum compound becomes noticeable at a considerably lower temperature (125°C to 150°C), and leads directly to the formation of aluminum, hydrogen and lithium hydride. Both compounds are soluble in diethyl ether, but the solubility of the aluminum compound is from seven to eight times as great as that of the borohydride. The former reacts completely and extremely rapidly when dropped into water; the reaction of the latter is slower and less nearly complete (It is to be noted, however, that lithium aluminum hydride may be safely handled, even in very humid air, probably because of the formation of a protective coating of aluminum hydroxide). Similar differences are observed in the reactions of the two compounds toward alcohols. Finally, lithium borohydride is inert toward liquid ammonia and toward amines, whereas the aluminum compound reacts with both.
The chemical properties of aluminum hydride are similar to those of lithium aluminum hydride, although the instability of the ether solution of the former limits its usefulness. Attention is here called to the possibility that the unstable ether solution of the compound may afford a means of following by molecular weight determinations the course of the polymerization assumed to be responsible for the stable, insoluble solid form of the substance.
The Preparation of Lithium Aluminum Hydride.A. Reagents.
Unless the lithium hydride is finer than 100 mesh, its reaction with aluminum chloride occurs very slowly. After weeks of refluxing 20 to 60 mesh hydride with a diethyl ether solution of aluminum chloride, only a slight surface reaction had occurred. The relatively coarse "crystalline lump" hydride, which seems to be the only form now commercially available, must be ground before use. The grinding and sifting of the material should be carried out in an atmosphere of dry nitrogen.
We have used samples of purity varying from 87 to 94% without significant differences in the results. The quantities of lithium hydride are stated in the description of the preparative procedures in terms of pure compound. The aluminum chloride was anhydrous and of reagent quality. Anhydrous diethyl ether of the usual commercial grade proved satisfactory.
B. Apparatus.
Most of the reactions were carried out in three-necked flasks fitted with a mercury-sealed stirrer, a reflux condenser, and a dropping funnel, such as are used for Grignard reactions. Usually an atmosphere of dry, carbon dioxide-free nitrogen was employed both during the reaction and in the filtrations, but these precautions were found not to be absolutely essential.
C. Procedure.
In a typical preparation, an excess of lithium hydride (23.5g or 2.96 moles) was added to a solution of 3.05g (0.08 mole) of lithium aluminum hydride in 30 ml of diethyl ether, and the mixture was stirred for a short time. After the addition of a further quantity of ether (200 ml), a solution of 71.2g (0.534 mole) of aluminum chloride in 300ml of ether was introduced at such a rate that boiling of the liquid in the reaction vessel was continuous. The mixture was stirred during this step, and for a short time after the reaction had apparently ceased The precipitated lithium chloride and the excess of lithium hydride were separated from the solution by passing the latter through a coarse sintered glass disk under nitrogen pressure.
An aliquot (6.049g) of the 462.5g of combined filtrate and washings was evaporated at atmospheric pressure until a thick sirup had formed The last of the ether was then removed in vacuo at 70°C. The resulting solid weighed 0.280g and consisted of lithium aluminum hydride of 95.4% purity according to an analysis carried out by dissolving an amount of the compound in anhydrous dioxane, and adding water drop by drop until gas evolution ceased. Lithium aluminum hydride hydrolyzes according to the following equation:
In the resulting solution, aluminum was determined as its oxide, and lithium as the sulfate. The quantity of the compound contained in the original solution was, therefore, 20.42g, of which 3.05g had been contained in the original reaction mixture. The net yield thus was 17.37g (0.458 mole) or 85.7% of the theoretical. It has been found that allowing the reaction to stand for a longer time before filtration improves the yield and increases the purity of the product (it may become as high as 99% without recrystallization). The bulk of the reaction product was usually not taken to dryness, since most of the reactions to be investigated were carried out in ethereal solutions. For determination of the concentrations of the solutions, measurement of the amount of hydrogen evolved by hydrolysis of an aliquot is adequate.
The addition of the small amount of previously prepared lithium aluminum hydride to the reaction mixture prevents a phenomenon which otherwise may be very troublesome. Without this addition, an initial reaction usually manifests itself by a slight rise in temperature, but soon either ceases entirely or becomes too slow to be appreciable. After an induction period, which may last for only a few minutes or may persist for hours, the reaction again sets in, this time with such vigor that it can usually not be controlled by cooling of the mixture. In the presence of a small initial quantity of lithium aluminum hydride, the reaction sets in at once at a rate controlled by the rate of addition of aluminum chloride. To obtain lithium aluminum hydride for this purpose when none is available from previous preparations, two procedures are illustrated by the following examples:
1. A small quantity of aluminum chloride (2.7g) was mixed with a 6-fold excess of lithium hydride (4g) under dry nitrogen in a small round bottomed flask, which was then attached through a standard ground glass joint to a vacuum system, and evacuated. About 15 ml of ether was condensed on the reaction mixture at liquid nitrogen temperature. The reaction, which began as the flask was warmed slowly, was allowed to proceed vigorously, but was kept under control by cooling the flask with liquid nitrogen from time to time. The reaction was usually completed in about five minutes. Filtration of the resulting mixture and removal of the solvent from the filtrate as previously described yielded a sample of lithium aluminum hydride adequate for initiation of the reaction of larger batches.
2. Solid lithium hydride (7.0g) was mixed with solid anhydrous aluminum chloride (15.95g) in a 500 ml flask previously flushed with dry nitrogen. Subsequent addition of 150 ml of dioxane caused the temperature to rise to 50°C. The mixture was refluxed for one-half hour, after which it was cooled, diluted with 135 ml of diethyl ether and refluxed again for another three hours. The resulting mixture was filtered. The solid obtained from the filtrate by the procedure used in the preceding examples seemed to contain only about 30% of lithium aluminum hydride, but nevertheless served to initiate the desired reaction.
Solubility of lithium aluminum hydride
The solubility of lithium aluminum hydride in grams per 100g of various ethers at 25°C are as follows: diethyl ether 25-30, tetrahydrofuran 13, dibutyl ether 2 and dioxane 0.1. Because of the difficulty of handling the extremely viscous solutions obtained in the first two solvents, the data are of only approximate character.
Reference: J. Am. Chem. Soc. 69, 1199 (1947)
http://lewis.armscontrolwonk.com/archive/7001/curious-case-of-red-mercury |
Saturday, September 5, 2015
source code NSA Keyscore
// START_DEFINITION
/**
* Fingerprint Tor authoritative directories enacting the directory protocol.
*/
fingerprint('anonymizer/tor/node/authority') = $tor_authority
and ($tor_directory or preappid(/anonymizer\/tor\/directory/));
// END_DEFINITION
// START_DEFINITION
/*
Global Variable for Tor foreign directory servers. Searching for potential Tor
clients connecting to the Tor foreign directory servers on ports 80 and 443.
*/
$tor_foreign_directory_ip = ip('193.23.244.244' or '194.109.206.212' or
'86.59.21.38' or '213.115.239.118' or '212.112.245.170') and port ('80' or
'443');
// END_DEFINITION
// START_DEFINITION
/*
this variable contains the 3 Tor directory servers hosted in FVEY countries.
Please do not update this variable with non-FVEY IPs. These are held in a
separate variable called $tor_foreign_directory_ip. Goal is to find potential
Tor clients connecting to the Tor directory servers.
*/
$tor_fvey_directory_ip = ip('128.31.0.39' or '216.224.124.114' or
'208.83.223.34') and port ('80' or '443');
// END_DEFINITION
// START_DEFINITION
requires grammar version 5
/**
* Identify clients accessing Tor bridge information.
*/
fingerprint('anonymizer/tor/bridge/tls') =
ssl_x509_subject('bridges.torproject.org') or
ssl_dns_name('bridges.torproject.org');
/**
* Database Tor bridge information extracted from confirmation emails.
*/
fingerprint('anonymizer/tor/bridge/email') =
email_address('bridges@torproject.org')
and email_body('https://bridges.torproject.org/' : c++
extractors: {{
bridges[] = /bridge\s([0-9]{1,3}\.[0-9]{1,3}\.[0-9]{1,3}\.[0-9]{1,3}):?([0-9]{2,4}?[^0-9])/;
}}
init: {{
xks::undefine_name("anonymizer/tor/torbridges/emailconfirmation");
}}
main: {{
static const std::string SCHEMA_OLD = "tor_bridges";
static const std::string SCHEMA_NEW = "tor_routers";
static const std::string FLAGS = "Bridge";
if (bridges) {
for (size_t i=0; i < bridges.size(); ++i) {
std::string address = bridges[i][0] + ":" + bridges[i][1];
DB[SCHEMA_OLD]["tor_bridge"] = address;
DB.apply();
DB[SCHEMA_NEW]["tor_ip"] = bridges[i][0];
DB[SCHEMA_NEW]["tor_port_or"] = bridges[i][1];
DB[SCHEMA_NEW]["tor_flags"] = FLAGS;
DB.apply();
}
xks::fire_fingerprint("anonymizer/tor/directory/bridge");
}
return true;
}});
// END_DEFINITION
// START_DEFINITION
/*
The fingerprint identifies sessions visiting the Tor Project website from
non-fvey countries.
*/
fingerprint('anonymizer/tor/torpoject_visit')=http_host('www.torproject.org')
and not(xff_cc('US' OR 'GB' OR 'CA' OR 'AU' OR 'NZ'));
// END_DEFINITION
// START_DEFINITION
/*
These variables define terms and websites relating to the TAILs (The Amnesic
Incognito Live System) software program, a comsec mechanism advocated by
extremists on extremist forums.
*/
$TAILS_terms=word('tails' or 'Amnesiac Incognito Live System') and word('linux'
or ' USB ' or ' CD ' or 'secure desktop' or ' IRC ' or 'truecrypt' or ' tor ');
$TAILS_websites=('tails.boum.org/') or ('linuxjournal.com/content/linux*');
// END_DEFINITION
// START_DEFINITION
/*
This fingerprint identifies users searching for the TAILs (The Amnesic
Incognito Live System) software program, viewing documents relating to TAILs,
or viewing websites that detail TAILs.
*/
fingerprint('ct_mo/TAILS')=
fingerprint('documents/comsec/tails_doc') or web_search($TAILS_terms) or
url($TAILS_websites) or html_title($TAILS_websites);
// END_DEFINITION
// START_DEFINITION
requires grammar version 5
/**
* Aggregate Tor hidden service addresses seen in raw traffic.
*/
mapreduce::plugin('anonymizer/tor/plugin/onion') =
immediate_keyword(/(?:([a-z]+):\/\/){0,1}([a-z2-7]{16})\.onion(?::(\d+)){0,1}/c : c++
includes: {{
#include
}}
proto: {{
message onion_t {
required string address = 1;
optional string scheme = 2;
optional string port = 3;
}
}}
mapper: {{
static const std::string prefix = "anonymizer/tor/hiddenservice/address/";
onion_t onion;
size_t matches = cur_args()->matches.size();
for (size_t pos=0; pos < matches; ++pos) {
const std::string &value = match(pos);
if (value.size() == 16)
onion.set_address(value);
else if(!onion.has_scheme())
onion.set_scheme(value);
else
onion.set_port(value);
}
if (!onion.has_address())
return false;
MAPPER.map(onion.address(), onion);
xks::fire_fingerprint(prefix + onion.address());
return true;
}}
reducer: {{
for (values_t::const_iterator iter = VALUES.begin();
iter != VALUES.end();
++iter) {
DB["tor_onion_survey"]["onion_address"] = iter->address() + ".onion";
if (iter->has_scheme())
DB["tor_onion_survey"]["onion_scheme"] = iter->scheme();
if (iter->has_port())
DB["tor_onion_survey"]["onion_port"] = iter->port();
DB["tor_onion_survey"]["onion_count"] = boost::lexical_cast(TOTAL_VALUE_COUNT);
DB.apply();
DB.clear();
}
return true;
}});
/**
* Placeholder fingerprint for Tor hidden service addresses.
* Real fingerpritns will be fired by the plugins
* 'anonymizer/tor/plugin/onion/*'
*/
fingerprint('anonymizer/tor/hiddenservice/address') = nil;
// END_DEFINITION
// START_DEFINITION
appid('anonymizer/mailer/mixminion', 3.0, viewer=$ascii_viewer) =
http_host('mixminion') or
ip('128.31.0.34');
// END_DEFINITION
Wednesday, August 5, 2015
Exploding chips could foil laptop thieves
A new way of making silicon explode could mean anyone trying to use a stolen laptop or mobile will be confronted by this message: “This machine is stolen and will self-destruct in ten seconds … “.
Until now scientists have only managed to make silicon go bang by mixing it with either liquid oxygen or nitric acid. But Michael Sailor and his colleagues at the University of California in San Diego have found a way to blow up silicon chips using an electrical signal.
They say their method could be used to fry circuitry in devices that fall into the wrong hands. For instance, the American spy plane impounded by China last year could have used it to destroy its secret electronics systems.
Sailor’s team hit upon this new way of exploding silicon when they applied the oxidising chemical gadolinium nitrate to a porous silicon wafer. As colleague Fred Mikulec used a diamond scribe to split the wafer it blew up in his face, giving Mikulec the shock of his life. Luckily, only a minute quantity of silicon was involved so it was a small bang. “It’s a bit like a cap in a cap gun going off,” says Sailor.
In a stolen mobile phone, the network would send a trigger signal to the part of the chip containing the gadolinium nitrate “detonator”, triggering the explosion. “We have shown that you can store this stuff and detonate it at will,” says Sailor.
Other applications suggested for the technology include testing for toxic substances in groundwater. The device could be used on the spot to burn minute samples on a disposable chip and analyse their chemical composition. Alternatively, it could be used as a fuel supply for microscopic machines etched onto silicon wafers, says Sailor.
Porous silicon-based explosive PATENT
US 7942989 B2
Until now scientists have only managed to make silicon go bang by mixing it with either liquid oxygen or nitric acid. But Michael Sailor and his colleagues at the University of California in San Diego have found a way to blow up silicon chips using an electrical signal.
They say their method could be used to fry circuitry in devices that fall into the wrong hands. For instance, the American spy plane impounded by China last year could have used it to destroy its secret electronics systems.
Sailor’s team hit upon this new way of exploding silicon when they applied the oxidising chemical gadolinium nitrate to a porous silicon wafer. As colleague Fred Mikulec used a diamond scribe to split the wafer it blew up in his face, giving Mikulec the shock of his life. Luckily, only a minute quantity of silicon was involved so it was a small bang. “It’s a bit like a cap in a cap gun going off,” says Sailor.
Fast burn
The gadolinium nitrate used the energy from the diamond scribe to oxidise the silicon fuel, which burns fast because its crystals have a large surface area. “The faster the burn, the bigger the bang,” explains Sailor. You would only need a tiny quantity of the chemical to do irreparable damage to delicate transistors, so it would be cheap and easy to add when the chips are being made.In a stolen mobile phone, the network would send a trigger signal to the part of the chip containing the gadolinium nitrate “detonator”, triggering the explosion. “We have shown that you can store this stuff and detonate it at will,” says Sailor.
Other applications suggested for the technology include testing for toxic substances in groundwater. The device could be used on the spot to burn minute samples on a disposable chip and analyse their chemical composition. Alternatively, it could be used as a fuel supply for microscopic machines etched onto silicon wafers, says Sailor.
Journal reference: Advanced Materials (vol 14, p 38)
US 7942989 B2
Resumo
An initiator explosive for detonating a second explosive that includes nanocrystalline silicon containing a plurality of pores and a solid state oxidant disposed within said pores.
CHANGE THE BIOS FOR THE MOBILE OPERATOR CAN'T MODIFY THE DETONATOR TIME
Create a USB Boot CD that can be used to boot Xubuntu 9.04 from USB flash drive on computers using a system BIOS that does not natively support booting from USB. The boot CD works by loading the initrd and vmlinuz kernel from the CD. Once the necessary USB drivers have been loaded, the boot CD proceeds to locate and load the filesystem from the USB flash drive. Because the USB driver modules are preloaded from the CD, the compressed filesystem can then be detected and loaded from the USB device even if your system BIOS does not support booting from USB.
Read the rest of this entry…
Read the rest of this entry…
Listed under Use a Boot CD to Boot from USB
Make a USB Boot CD for Kubuntu 9.04
This tutorial explains how to create a USB Boot CD that can be used to boot Kubuntu 9.04 from a USB flash drive on computers utilizing a system BIOS that does not natively support booting from USB. Kubuntu is a derivative of Ubuntu that uses the KDE desktop environment instead of Gnome. The USB boot CD created using this tutorial launches the initrd and vmlinuz kernel from the CD along with the necessary USB drivers, and then proceeds to locate the filesystem on the USB drive. Because the USB driver modules are preloaded from the initrd on the CD, the compressed filesystem can then be detected and loaded from the USB device.
Read the rest of this entry…
Read the rest of this entry…
Listed under Use a Boot CD to Boot from USB
Make a USB Boot CD for Kubuntu 8.10
This USB Boot CD can be used to boot Kubuntu 8.10 from a USB flash drive on computers with a BIOS that does not support booting from USB (including the Apple Mac, Macbook and, Macbook Pro). Kubuntu is a derivative of Ubuntu that uses the KDE desktop environment instead of Gnome. This boot CD loads the initrd and vmlinuz kernel from the CD and then loads the necessary USB drivers, proceeding to locate and extract the filesystem from the USB flash drive.
Read the rest of this entry…
Read the rest of this entry…
Listed under Use a Boot CD to Boot from USB
Make a USB Boot CD for Xubuntu 8.10
Much like a USB Boot CD that can be used to boot Ubuntu 8.10 from USB, this USB Boot CD can be used to boot a prepared Xubuntu 8.10 USB flash drive on computers containing a system BIOS that does not natively support booting from USB. The boot CD works by loading the initrd and vmlinuz kernel from the CD. Once the necessary USB drivers have been loaded, the boot CD proceeds to locate and load the filesystem from the USB flash drive.
Read the rest of this entry…
Read the rest of this entry…
Listed under Use a Boot CD to Boot from USB
Make a USB Boot CD for Ubuntu 8.10
This USB Boot CD can be used to boot a Ubuntu 8.10 USB flash drive on computers with a BIOS that does not natively support booting from USB. The boot CD contains a grub bootloader that loads the initrd and vmlinuz kernel from the CD and then proceeds to locate the filesystem on the USB flash drive. Because the USB drivers are preloaded from the initrd on the CD, the USB flash drive can then easily be detected.
Read the rest of this entry…
Read the rest of this entry…
Listed under Use a Boot CD to Boot from USB
How to Access BIOS
How to Access BIOS. Computer and motherboard manufacturers and BIOS suppliers may use varying keyboard keys or key combinations that can be pressed during system post to access your system BIOS. Unfortunately there is no standard method to universally access or enter a motherboard BIOS. The following is a list of some popular BIOS suppliers, Computer Vendors and the keyboard key combinations that have been known to work with them.
Read the rest of this entry…
Read the rest of this entry…
Listed under USB BIOS boot options
Booting Linux using USB-ZIP on older systems
If you have an older computer system, your BIOS might not support USB-HDD boot. In this case, it may still be possible to boot Linux from USB if your BIOS does list USB-ZIP as a boot option. In order for this to happen, we need to trick the BIOS into thinking that the USB flash drive is a zip drive.
We can trick the BIOS by modifying the number of heads and sectors being displayed from the USB flash device to match that of a zip drive. Then we partition the drive using partition 4 (the partition that zip drives typically use). For this tutorial we will use the mkdiskimage application that comes with syslinux.
Read the rest of this entry…
http://www.pendrivelinux.com/tag/bios/
Having the correct balance of fuel and oxidizer is key to having a stable burn in a thermite reaction. The thermite recipe for red iron oxide and aluminum is 3 parts iron oxide red to 1 part aluminum fuel. In pyrotechnic compositions the finer the ingredients and more well mixed they are, generally the easier it is to ignite, and the faster the reaction will progress.
So our goals in selecting thermite ingredients are ease of ignition and a stable, non-explosive burn.
For this example, we are using Skylighter's -325 mesh red iron oxide and Skylighter's -325 mesh bright aluminum, which has an average particle size of 45 microns.
Thermite#1 Mixture
To make a 4 ounce batch of thermite, first weigh out 3 ounces of red iron oxide. Then weigh out 1 ounce of aluminum powder. Dump them into a plastic tub, and put the top on. Shake them until the color is consistent throughout.
Then pass the mix through a 10-mesh or finer screen. If there are any clumps break them up with your fingers. Screen the mix two more times, or more, until you have a uniformly colored powder.
This can be a dusty process, so be sure and do it an area where the aluminum dust will not cause a problem. Do not try this where air is moving. The better you mix the ingredient, the faster and hotter the reaction will be. Thermite needs a very high temperature to ignite it.
Your thermite kit comes with 6 gold sparklers. The iron in them burns at around 1800 degrees F.
Here's how to use sparklers to ignite your thermite. Place your thermite mix in a plastic tub. Make a hole near the bottom on the side of the tub big enough for a sparkler to go through.
Push 1 to 2 of inches of the sparkler through the hole in the side of the tub into the mix inside. Leave at least 2-3 inches of your sparkler sticking out. Place the plastic tub on a metal surface that want to burn through. Light the sparkler, and get back 40 or 50 feet.
WARNING: Do NOT attempt to ignite your thermite by dipping your sparkler into the mix by hand. The mix can and does ignite violently, and will spray you with burning metal.
WARNING: A violent ignition of thermite can throw molten metal in all directions. Do not stand close to the mix when it is igniting or burning.
TIP: If you have trouble igniting your thermite with a sparkler, try mixing some magnesium powder or chips into a small amount of thermite mix. Then you should be able to ignite the magnesium mix using your sparkler.
WARNING: Thermite burns extremely hot and produces molten iron slag that can melt though a car's engine block! Burning thermite can spatter molten iron a long way from the burning pile. Stay as far back as you can. And make sure there is nothing nearby that can catch fire. Start your thermite experiments with small amounts at first, until you understand how it behaves, and how far it will throw molten metal slag.
WARNING: You cannot extinguish a thermite fire with water. Do not attempt to put the fire out with water, or you may have a violent, steam explosion, which can throw molten slag as well. Likewise, do not use any wet materials to attempt to extinguish the fire.
http://www.skylighter.com/fireworks/how-to-make/Thermite.asp
FOR SALE SEARCH FOR THE CAS NUMBER:
Metal Nano Powder
We can trick the BIOS by modifying the number of heads and sectors being displayed from the USB flash device to match that of a zip drive. Then we partition the drive using partition 4 (the partition that zip drives typically use). For this tutorial we will use the mkdiskimage application that comes with syslinux.
Read the rest of this entry…
http://www.pendrivelinux.com/tag/bios/
Making Thermite Using Red Iron Oxide
Having the correct balance of fuel and oxidizer is key to having a stable burn in a thermite reaction. The thermite recipe for red iron oxide and aluminum is 3 parts iron oxide red to 1 part aluminum fuel. In pyrotechnic compositions the finer the ingredients and more well mixed they are, generally the easier it is to ignite, and the faster the reaction will progress.
So our goals in selecting thermite ingredients are ease of ignition and a stable, non-explosive burn.
For this example, we are using Skylighter's -325 mesh red iron oxide and Skylighter's -325 mesh bright aluminum, which has an average particle size of 45 microns.
| Ingredient | Parts by Wt. | Parts by % |
| Red iron oxide -325 mesh | 3 | 75 |
| Aluminum flake -325 mesh | 1 | 25 |
| Total | 100% |
To make a 4 ounce batch of thermite, first weigh out 3 ounces of red iron oxide. Then weigh out 1 ounce of aluminum powder. Dump them into a plastic tub, and put the top on. Shake them until the color is consistent throughout.
Then pass the mix through a 10-mesh or finer screen. If there are any clumps break them up with your fingers. Screen the mix two more times, or more, until you have a uniformly colored powder.
This can be a dusty process, so be sure and do it an area where the aluminum dust will not cause a problem. Do not try this where air is moving. The better you mix the ingredient, the faster and hotter the reaction will be. Thermite needs a very high temperature to ignite it.
Your thermite kit comes with 6 gold sparklers. The iron in them burns at around 1800 degrees F.
Here's how to use sparklers to ignite your thermite. Place your thermite mix in a plastic tub. Make a hole near the bottom on the side of the tub big enough for a sparkler to go through.
Push 1 to 2 of inches of the sparkler through the hole in the side of the tub into the mix inside. Leave at least 2-3 inches of your sparkler sticking out. Place the plastic tub on a metal surface that want to burn through. Light the sparkler, and get back 40 or 50 feet.
WARNING: Do NOT attempt to ignite your thermite by dipping your sparkler into the mix by hand. The mix can and does ignite violently, and will spray you with burning metal.
WARNING: A violent ignition of thermite can throw molten metal in all directions. Do not stand close to the mix when it is igniting or burning.
TIP: If you have trouble igniting your thermite with a sparkler, try mixing some magnesium powder or chips into a small amount of thermite mix. Then you should be able to ignite the magnesium mix using your sparkler.
WARNING: Thermite burns extremely hot and produces molten iron slag that can melt though a car's engine block! Burning thermite can spatter molten iron a long way from the burning pile. Stay as far back as you can. And make sure there is nothing nearby that can catch fire. Start your thermite experiments with small amounts at first, until you understand how it behaves, and how far it will throw molten metal slag.
WARNING: You cannot extinguish a thermite fire with water. Do not attempt to put the fire out with water, or you may have a violent, steam explosion, which can throw molten slag as well. Likewise, do not use any wet materials to attempt to extinguish the fire.
http://www.skylighter.com/fireworks/how-to-make/Thermite.asp
FOR SALE SEARCH FOR THE CAS NUMBER:
Metal Nano Powder
Thursday, July 30, 2015
Optical Emission Security – Frequently Asked Questions
Markus Kuhn In the paperOptical Time-Domain Eavesdropping Risks of CRT Displays,I describe a new eavesdropping technique that reconstructs text on computer screens from diffusely reflected light. This publication resulted in some wide media attention (BBC, New Scientist,Wired, Reuters, Slashdot). Here are answers to some of the questions I have received, along with some introductory information for interested readers looking for a more highlevel summary than the full paper, which was mainly written for an audience of hardware-security and optoelectronics professionals. Q: How does this new eavesdropping technique work? To understand what is going on, you have to recall how a cathode-ray display works. An electron beam scans across the screen surface at enormous speed (tens of kilometers per second) and targets one pixel after another. It targets this way tens or hundreds of millions of pixels every second to convert electron energy into light. Even though each pixel shows an afterglow for longer than the time the electron beam needs to refresh an entire line of pixels, each pixel is much brighter while the e-beam hits it than during the remaining afterglow. My discovery of this very short initial brightness in the light decay curve of a pixel is what makes this eavesdropping technique work. An image is created on the CRT surface by varying the electron beam intensity for each pixel. The room in which the CRT is located is partially illuminated by the pixels. As a result, the light in the room becomes a measure for the electron beam current. In particular, there is a little invisible ultrafast flash each time the electron beam refreshes a bright pixel that is surrounded by dark pixels on its left and right. So if you measure the brightness of a wall in this room with a very fast photosensor, and feed the result in another monitor that receives the exact same synchronization signals for steering its electron beam, you get to see an image like this:
2002 IEEE Symposium on Security and Privacy, Berkeley, California, May 2002.
- The eavesdropped videosignal is periodic over at least a few seconds, therefore periodic averaging over a few hundred frames can help significantly to reduce the noise.
- If you know exactly what font is used, many of the equalization and symbol detection techniques used in modems or pattern recognition applications can be applied to recover the text (remote optical character recognition).
- Optical filters can eliminate other colours from background light.
- A large sensor aperture (telelens, telescope) can improve the photon count.
- Reception is difficult if not impossible from well-lit rooms, in which CRTs do not make a visible contribution to the ambient illumination. Don't work in the dark.
- No not assume that etched or frosted glass surfaces prevent this technique if there is otherwise a direct line of sight to the screen surface.
- This particular eavesdropping technique is not applicable to LCDs and other flat-panel displays that refresh all pixels in a row simultaneously.
- Make sure, nobody can install eavesdropping equipment within a few hundred meters line-of-sight to your window.
- Use a screen saver that removes confidential information from the monitor in your absence.
created 2002-03-05 – last modified 2004-11-29 – http://www.cl.cam.ac.uk/~mgk25/emsec/optical-faq.html
Subscribe to:
Comments (Atom)